WHO Flags Toxic Cough Syrups In India After Child Deaths
International
oi-Prakash KL

The World Health Organization (WHO) has sounded a global alarm over three Indian-made cough syrups found to be contaminated with a lethal chemical compound, following the deaths of multiple children in Madhya Pradesh. The agency urged countries to immediately report any detection of these products within their borders.
Medicines Under Scrutiny
The syrups identified are:
The World Health Organization (WHO) alerted countries about three Indian-made cough syrups—Coldrif, Respifresh TR, and ReLife—found contaminated with diethylene glycol (DEG), a toxic chemical, following deaths of children in Madhya Pradesh; at least 22 children died after consuming the Coldrif syrup made by Sresan Pharmaceutical, whose manufacturing license was revoked and owner G Ranganathan was arrested.
-
Coldrif
by
Sresan
Pharmaceutical
-
Respifresh
TR
by
Rednex
Pharmaceuticals
-
ReLife
by
Shape
Pharma
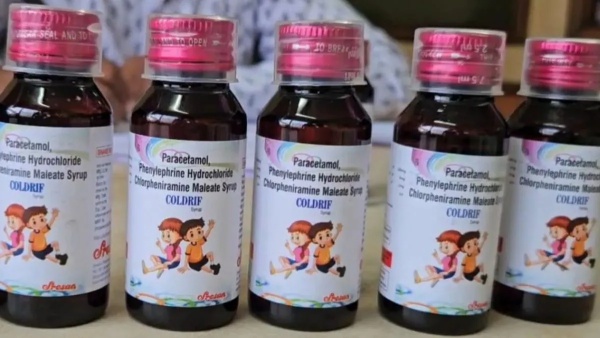
According to the WHO, these products contain dangerously high levels of diethylene glycol (DEG), a toxic solvent known to cause kidney failure and death. Lab tests revealed DEG concentrations nearly 500 times the permissible limit.
Tragedy in Chhindwara
India's Central Drugs Standard Control Organization (CDSCO) confirmed that the syrups were consumed by children under five in Chhindwara, Madhya Pradesh. At least 22 children, mostly from Parasia village, died after ingesting Coldrif syrup.
Manufacturer Crackdown Sresan Pharmaceuticals, the Tamil Nadu-based company behind Coldrif, has had its manufacturing license revoked. Its owner, G Ranganathan, was arrested amid growing outrage. The Indian government has launched inspections of other pharmaceutical units in the region
Global Implications
WHO has requested clarity on the international distribution of the contaminated syrups. Indian authorities assured that none of the affected products were exported, and the U.S. Food and Drug Administration confirmed that the syrups were not shipped to the United States.
Government Advisory
In response to the crisis, the Indian government issued a nationwide advisory urging healthcare providers to avoid prescribing cough syrups to children under two years old and to exercise caution for those under five. The advisory aims to prevent further tragedies linked to pediatric medication misuse.
